A theoretical study of the gas phase (proton affinity) and aqueous (pKa) basicity of a series of 150 pyrazoles†
Abstract
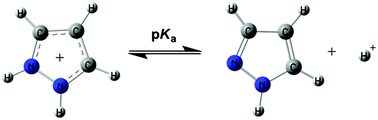
This study deals with the basicity, both in the gas phase and in an aqueous solution, of a set of 150 pyrazoles covering a range of about 200 kJ mol−1 in proton affinity and 10–15 pKa units. There are 63 NH-pyrazoles, in many cases, with two different tautomers and 87 N-substituted pyrazoles. The gas phase results are well reproduced by DFT theoretical calculations when the less stable tautomers are removed. For the pKa values to be reproduced adequately by calculations, some of the tautomers need to be removed, and the use of dummy variables accounting for the protonation on exocyclic amine groups is required and the conformation of the 5-phenyl groups need to be considered. Using these restrictions a large number of unknown pKas can be predicted and a few errors corrected.

 Please wait while we load your content...
Please wait while we load your content...