Structural investigation on toluene-3,4-dithiolatoantimony(iii) alkyldithiocarbonate complexes: thermal, powder XRD and biological studies†
Abstract
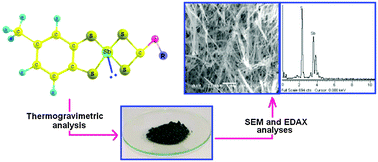
Toluene-3,4-dithiolatoantimony(III) complexes with alkyldithiocarbonates of the type CH3(C6H3)S2SbS2COR [where R = CH3 (1), C2H5 (2), iC3H7 (3), nC3H7 (4), iC4H9 (5) and nC4H9 (6)] have been synthesized by the reaction of toluene-3,4-dithiolatoantimony(III) chloride with potassium alkyldithiocarbonates in 1 : 1 molar stoichiometry. The nature of bonding of dithiocarbonate ligand to antimony metal is anisobidentate, shown by IR and carbon resonance (13C NMR) spectral analyses. These complexes have nano ranged particle size (4.39–7.29 nm), and the lower (monoclinic) symmetry crystal system supports the distorted geometry of the synthesized complexes. After the thermal decomposition of these types of complexes in an inert atmosphere, we have obtained antimony sulfide as a final thermal decomposition product. It was further accomplished by SEM and EDAX studies. Toluene-3,4-dithiol ligand has a stronger chelating nature than xanthate ligand, which has been confirmed by ESI mass spectra. The antimony to sulfur bond has been shown to have greater antimicrobial activities than free ligands and standard drugs (chloramphenicol or terbinafine).

 Please wait while we load your content...
Please wait while we load your content...