Decomposition of benzoylthioureas into benzamides and thiobenzamides under solvent-free conditions using iodine–alumina as the catalyst and its mechanistic study by density functional theory†
Abstract
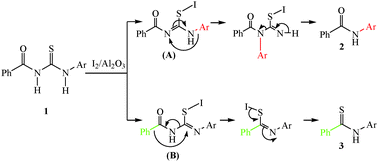
The breaking down of benzoylthioureas to benzamides and thiobenzamides in a single route using iodine–alumina as the catalyst under solvent-free conditions is highlighted. Results show that when an electron donating group, such as the methyl or methoxy group, is at the para-position of the aryl group of 1, benzamide (2) is the favoured product. When an electron withdrawing group, such as the chlorine or nitro group, is at the para-position of the aryl group of 1, thiobenzamide (3) is the favoured product. From the study of the reaction mechanism, it may be postulated that the formation of benzamide is due to the migration of the aryl group, whereas the formation of thiobenzamide may be due to the migration of the phenyl group. Thus, a new method for the formation of benzamides and thiobenzamides was developed.

 Please wait while we load your content...
Please wait while we load your content...