Uranium extraction using a magnetic CoFe2O4–graphene nanocomposite: kinetics and thermodynamics studies
Abstract
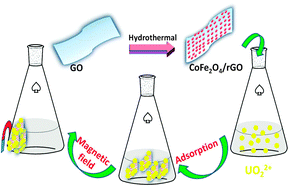
A novel magnetic nanocomposite (CoFe2O4–rGO), consisting of reduced graphene oxide (rGO) and CoFe2O4 nanoparticles, was fabricated. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD) and a vibrating sample magnetometer (VSM) were used to characterize the CoFe2O4–rGO. The results indicate that CoFe2O4 nanoparticles have been successfully installed on the surface of rGO. Uranium adsorption (from synthetic solutions) has been investigated in batch systems. Moreover, the effects of different experimental parameters, such as initial solution pH (controlled with nitric acid), equilibration time, initial uranium concentration and temperature, on sorption performance have been investigated. The results show that the kinetic data can be efficiently modelled using the pseudo-second-order equation. Furthermore, the Langmuir equation fits the sorption isotherms well. In addition, the values of thermodynamic parameters (ΔG° and ΔH°) show that the process is spontaneous and exothermic. These experimental results demonstrate the potential application of CoFe2O4–rGO in radionuclide cleanup.

 Please wait while we load your content...
Please wait while we load your content...