Tolane-based bent bolaamphiphiles forming liquid crystalline hexagonal honeycombs with trigonal symmetry†
Abstract
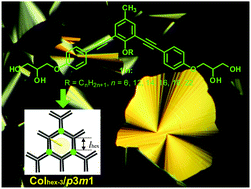
Bolaamphiphiles consisting of a bent 1,3-bis(phenylethynyl)benzene core with two terminal glycerol units, a lateral n-alkyl chain in the bay-position and a methyl group at the apex of the central 1,3-substituted benzene ring have been synthesized via Sonogashira coupling reactions as key steps. The thermotropic and solvent-modified or solvent-induced liquid crystalline (LC) phases of these compounds were investigated by POM, DSC and X-ray scattering. Compounds with medium alkyl chain length form a hexagonal honeycomb LC phase with non-centrosymmetric trigonal p3m1 symmetry as a monotropic LC phase. The addition of water increases the LC phase range, leading to enantiotropic p3m1 phases and induces LC phases for the non-mesomorphic compounds with shorter and longer lateral alkyl chains.

 Please wait while we load your content...
Please wait while we load your content...