Induced oxygen vacancies and their effect on the structural and electrical properties of a fluorite-type CaZrO3–Gd2Zr2O7 system
Abstract
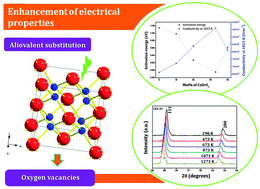
Solid oxide materials were prepared via a solid state reaction route with a stoichiometry composition: x% of CaZrO3 and (100 − x)% of Gd2Zr2O7 (x = 10, 20, 33.3, 40). Powder X-ray diffraction under ambient and high-temperature conditions, scanning electron microscopy, transmission electron microscopy and ac impedance spectroscopy techniques were used to analyze and correlate the structural and electrical properties of the materials. The aliovalent substitution of Gd3+ by Ca2+ allowed the creation of more oxygen vacancies in the lattice of these compositions. This resulted in a progressive decrease in the lattice parameter and an increase in the thermal expansion coefficient. The induced oxygen vacancies provided a lower energy barrier for ionic diffusion and hence led to an increase in conductivity which was found to show a maximum value of 3.59 × 10−3 S cm−1 at 1023 K for x = 33.3. The high ionic conductivity value measured in this study indicates that solid solutions of this kind are promising as good ionic conductors.

 Please wait while we load your content...
Please wait while we load your content...