Aromatic oligoamides with increased backbone flexibility: improved synthetic efficiencies, solvent-dependent folding and cooperative conformational transitions†
Abstract
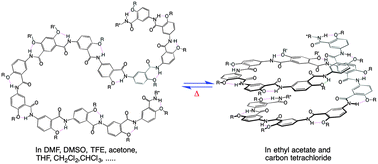
Aromatic oligoamides 2a, 2b, and 2c of increasing chain lengths were prepared and further characterized for their folding behaviour. These oligomers were derived by relaxing the backbone-constraint of a series of oligoamides that fold into well-defined conformations. With their backbones of increased flexibility, the resultant 2a–c were found to form with considerably improved efficiencies, and undergo highly cooperative folding that depends on chain length, solvents, and temperature.
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...