Synthesis of a double-stranded spiroborate helicate bearing stilbene units and its photoresponsive behaviour†
Abstract
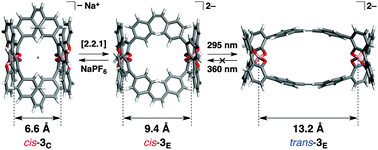
A novel spiroborate-based double-stranded helicate bearing photoresponsive cis-stilbene units in the middle (cis-3) was successfully synthesised from the corresponding cis-stilbene-bound tetraphenol strand in the presence of NaBH4, whereas the tetraphenol strands with a trans-stilbene or trans-azobenzene unit did not form such a double-stranded helicate. The 1H NMR and NOESY experiments revealed that cis-3 adopted contracted (cis-3C) and extended (cis-3E) forms under equilibrium in CD3CN at 25 °C. The contracted cis-3C that accommodated a Na+ ion in the center showed almost reversible extension and contraction motions by removal and addition of a Na+ ion. The cis-to-trans photoisomerisation of the extended cis-3E with UV light (295 nm) further induced an extension of the helicate, producing a mixture of cis,trans-3E and trans-3E helicates in the photostationary state. However, trans-to-cis photoisomerisation of the trans-mixtures using UV light (360 nm) was irreversible in this system and produced the photooxidated aldehyde species (trans-4), resulting from the photo-cleavage of the trans-stilbene moieties of trans-3E.
- This article is part of the themed collection: Foldamer Chemistry


 Please wait while we load your content...
Please wait while we load your content...