O-Arylation with nitroarenes: metal-catalyzed and metal-free methodologies
Abstract
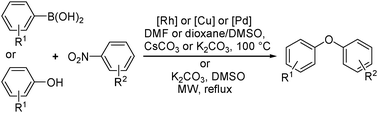
In this article, we focus on the introduction of nitroarene as an alternative electrophile for Caryl–O cross-coupling chemistry. In polar aprotic solvents and in the presence of a base at elevated temperature nitroarene undergoes cross-coupling with arylboronic acids or phenols under metal or metal-free reaction conditions. Compared to the conventional aryl halides, nitroarene provides highly attractive and environmentally friendly options for the synthesis of diaryl ether derivatives.

 Please wait while we load your content...
Please wait while we load your content...