Highly selective and effective mercury(ii) fluorescent sensors†
Abstract
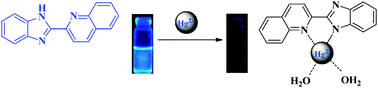
A novel mercury non-sulfur and simple fluorescent chemosensor L based on the benzimidazole group and quinoline group as the fluorescence signal group has been designed and synthesized. The receptor could instantly detect the Hg2+ cation over other cations such as Fe3+, Ag+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, and Mg2+ by fluorescence spectroscopy changes in H2O/DMSO (1 : 9, v/v) solution with specific selectivity and high sensitivity. The fluorescence color of the solution containing sensor L showed a remarkable change from blue to colorless only after the addition of Hg2+ in aqueous media while other cations did not cause obvious color changes. Moreover, further study demonstrates that the detection limit on the fluorescence response of the sensor to Hg2+ is down to 9.56 × 10−9 M, which is far lower than the maximum level for mercury of 0.01 M in drinking water, from EPA guidelines. Test strips based on L were fabricated, which could act as convenient and efficient Hg2+ test kits. Thus, the probe should have potential applications in an aqueous environment for the monitoring of mercury.

 Please wait while we load your content...
Please wait while we load your content...