Nucleoside bearing boron clusters and their phosphoramidites – building blocks for modified oligonucleotide synthesis†
Abstract
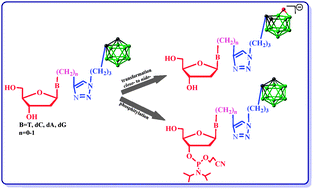
This paper describes a general method for the synthesis of four canonical nucleosides T, dC, dA and dG and their phosphoramidites suitable for automated synthesis of DNA modified with a carborane cage. A boron cluster in the form of an electroneutral, lipophilic 1,2-dicarba-closo-dodecaborane (C2B9H11) or negatively charged, redox-active 7,8-dicarba-nido-undecaborate ion (C2B9H12(−1)) was used as a modifying unit. The method is based on the “click chemistry” type Huisgen–Sharpless–Meldal reaction. All boron cluster-nucleoside conjugates have been characterized electrochemically; they have shown different redox potentials allowing for selective electrochemical identification of individual nucleosides in the mixture. There is also the first description of the crystallographic structure of the boron cluster-nucleoside conjugate: N3-{[(o-carboran-1-yl)propyl]-1N-1,2,3-triazol-4-yl}methylenethymidine.

 Please wait while we load your content...
Please wait while we load your content...