Tandem cyclocondensation-Knoevenagel–Michael reaction of phenyl hydrazine, acetoacetate derivatives and arylaldehydes†
Abstract
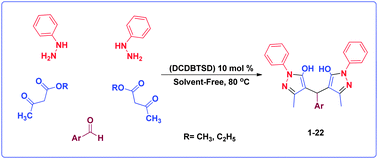
In this work, a novel N-bromo sulfonamide reagent, namely N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD), is synthesized and fully characterized by IR, UV, 1H and 13C NMR, XRD, TG/DTG as well as mass spectra. The presented N-bromo sulfonamide is used as a new and highly efficient catalyst for the synthesis of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s via one-pot pseudo five component condensation reaction of phenylhydrazine, acetoacetate derivatives and arylaldehydes. Mechanistically, Br+ ions, which are generated by the catalyst, can catalyze this reaction in neutral media. This methodology offers several advantages, such as use of non-toxic and inexpensive materials, high yields, short reaction times and clean workup.

 Please wait while we load your content...
Please wait while we load your content...