Easily-soluble heteroacene bis(benzothieno)silole derivatives for sensing of nitro explosives†
Abstract
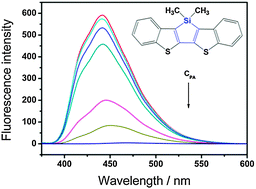
Three new heteropentacene derivatives based on bis(benzothieno)silole were synthesized. The XRD characterization reveals that the crystal structures of the heteropentacenes can be significantly influenced by the methyl substitution. The optical and electrochemical properties were investigated and the experimental results confirm that the silole motif lowers the LUMO level of the heteropentacenes. Preliminary results show that the insertion of silole ring systems into rigid heteropentacenes leads to an improved solubility and high fluorescence quantum yield up to 0.74 in solution, which indicates the potential of these new silole-containing compounds for photonic and photoelectric applications. The strong and sensitive fluorescence quenching behavior towards picric acid (PA) demonstrates that the silole-containing heteropentacene derivatives are also promising fluorescent sensor materials for detecting the nitro-containing explosives.

 Please wait while we load your content...
Please wait while we load your content...