Task specific ionic liquids as polarity shifting additives of common organic solvents†
Abstract
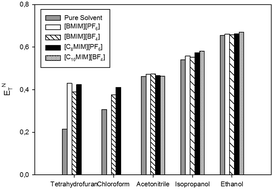
The effect of addition of small quantities of room temperature ionic liquids (RTILs) to common organic solvents (acetonitrile, ethanol, tetrahydrofuran, isopropanol and chloroform) on polarity is explored. It is found that solvent polarity always increases with addition of RTILs following a linear increase until reaching a plateau. [C2OHMIM][PF6], [C10MIM][BF4] and [EMIM][NTf2] have a dramatic influence on the polarity of acetonitrile, displacing it by more than 0.15 on the normalized polarity scale (ENT). When the same RTIL ([BMIM][BF4]) is added in the same quantity to different solvents, it is observed that the polarity obtained follows generically the same trend as the polarities of the pure solvents, with the exception of chloroform, which has a higher polarity than tetrahydrofuran when pure, but lower when [BMIM][BF4] is added.

 Please wait while we load your content...
Please wait while we load your content...