The facile coupling of carbon monochalcogenides to ethenedichalcogenone ligands in binuclear iron carbonyl derivatives: a theoretical study†
Abstract
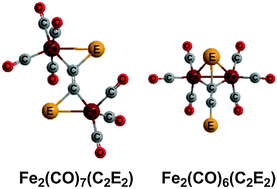
The lowest energy Fe2(CO)n(CE)2 structures (E = S, Se, Te; n = 8, 7, 6) do not have separate CE ligands but instead have coupled C2E2 ligands functioning as four to six electron donors to the pair of iron atoms. The exothermicity of such coupling reactions as a function of the chalcogen E increases in the sequence S < Se < Te. However, the monomers Fe(CO)4(CE) are stable species owing to the high activation energies for the coupling reactions. Thus, the thiocarbonyl Fe(CO)4(CS) is a stable experimentally known complex. However, the coupled Fe2(CO)7(C2E2) structures are preferred both thermodynamically and kinetically over isomeric Fe2(CO)7(CE)2 structures with discrete CE ligands. For the CS ligand, the previously optimized lowest energy Fe2(CO)n(CS)2 structures (n = 7, 6) with discrete CS ligands are now found to lie ∼11 kcal mol−1 (n = 7) and ∼30 kcal mol−1 (n = 6) in energy above the lowest energy isomeric Fe2(CO)n(C2S2) structures with coupled C2E2 ligands.

 Please wait while we load your content...
Please wait while we load your content...