Electrochemistry and structural properties of new mixed ligand nickel(ii) complexes based on thiosemicarbazone†
Abstract
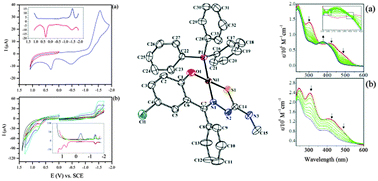
Mixed ligand nickel(II) complexes of 5-chloro-2-hydroxybenzophenone-N-R-thiosemicarbazone (R: –CH3 (L1), –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (LII)) and triphenylphosphine were synthesized. The structures of the complexes were characterized by elemental analysis, IR, 1H and 31P NMR spectroscopy, conductivity, electrochemical and magnetic moment measurements, and single-crystal X-ray diffraction technique. The two nickel(II) complexes have a square planar geometry containing O, N, and S atoms of the thiosemicarbazone, and the P atom of triphenylphosphine. The electrochemical behaviors of the thiosemicarbazone ligands and the nickel complexes were studied using cyclic voltammetry and square wave voltammetry. The redox processes of the compounds were significantly influenced by the central metal ions and the nature of the substituents on the thiosemicarbazones, which are the important factors in controlling the redox properties. In situ spectroelectrochemical studies were employed to determine the colors and spectra of the electro-generated species of the complexes.
CH2 (LII)) and triphenylphosphine were synthesized. The structures of the complexes were characterized by elemental analysis, IR, 1H and 31P NMR spectroscopy, conductivity, electrochemical and magnetic moment measurements, and single-crystal X-ray diffraction technique. The two nickel(II) complexes have a square planar geometry containing O, N, and S atoms of the thiosemicarbazone, and the P atom of triphenylphosphine. The electrochemical behaviors of the thiosemicarbazone ligands and the nickel complexes were studied using cyclic voltammetry and square wave voltammetry. The redox processes of the compounds were significantly influenced by the central metal ions and the nature of the substituents on the thiosemicarbazones, which are the important factors in controlling the redox properties. In situ spectroelectrochemical studies were employed to determine the colors and spectra of the electro-generated species of the complexes.

 Please wait while we load your content...
Please wait while we load your content...