Controllable synthesis of spherical anatase mesocrystals for lithium ion batteries
Abstract
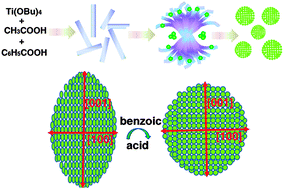
Spherical anatase mesocrystals have been successfully prepared in an acetic acid–tetrabutyl titanate–benzoic acid system via a facile solvothermal method. Based on the results of time-dependent experiments, a growth process is proposed for the spherical mesocrystals, which includes the formation of flower-like intermediates, the hydrolysis of these intermediates, and the precipitation and self-assembly of TiO2 nanocrystals. Benzoic acid plays an important role in the formation of the spherical morphology. Well-defined spherical anatase mesocrystals can be obtained only when the benzoic acid content is >4.5 g. As a result of their unique architecture (high crystallinity and a large specific surface area), the Li ion storage capabilities of these spherical mesocrystals were also investigated. The first discharge capacity is 314.5 mA h g−1 and the corresponding charge capacity is 224.3 mA h g−1, leading to a Coulombic efficiency of 71.3% at 0.2 C. Even under high current densities, such as 2 and 5 C, this anode material retains a good cycling stability and very high specific capacity.

 Please wait while we load your content...
Please wait while we load your content...