Self-assembly of crown ether-based amphiphiles for constructing synthetic ion channels: the relationship between structure and transport activity†
Abstract
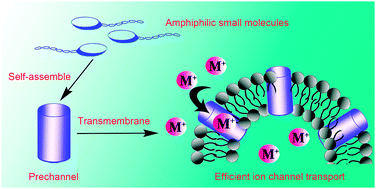
Synthetic ion channels arise great interest due to their ability to mimic the biological functions of natural ion channels. Although a number of synthetic ion channels have been reported and have displayed biomedical applications, the design criteria for efficient synthetic ion channels remain essentially obscure. According to the structure framework of our previously prepared synthetic ion channel (compound 1), in this study, a series of amphiphilic small molecules (compounds 2, 3a–3b, 4a–4c and 5) with structural simplicity were designed to explore the relationship between the molecular structure and the transport activity in this system. By adjusting the substituted functional moieties in the four building blocks, it was concluded that appropriate supramolecular interactions and reasonable water solubility favored improvement of the transport activity of the channels. Finally, compound 6 with an optimized structure was prepared using favorable crown ether, amide, diethylene glycol-substituted benzene, and dodecyl hydrophobic moieties as building blocks. This compound exhibited efficient channel transport with significantly improved transport activity, in which the EC50 value was 3 times lower than that of compound 1. These results provide some useful experience for developing excellent synthetic ion channels based on artful regulation on the structures of facially synthetic small molecules.

 Please wait while we load your content...
Please wait while we load your content...