Synthesis, identification and in vitro biological evaluation of some novel 5-imidazopyrazole incorporated pyrazoline and isoxazoline derivatives†
Abstract
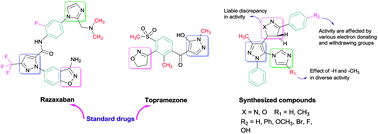
In the present study, novel combinatorial libraries of substituted pyrazolines 6a–l and isoxazolines 7a–l have been synthesized via the reactions of chalcones with the hydrazine hydrate and hydroxylamine hydrochloride in ethanol. The title compounds were screened for their preliminary in vitro antibacterial activity against a panel of pathogenic strains, in vitro antituberculosis activity against Mycobacterium tuberculosis H37Rv, in vitro antimalarial activity against Plasmodium falciparum and in vitro antioxidant activity by the ferric-reducing antioxidant power method. Compounds 6k, 6l, 7h and 7k exhibited excellent antibacterial activity and a few of them exhibited moderate antituberculosis activity compared with the first line drugs. Half of the compounds exhibited terrific antimalarial activity and the majority of compounds showed highest antioxidant potency.

 Please wait while we load your content...
Please wait while we load your content...