Anion influence on transformations of a nonporous 3D to porous 3D coordination polymer†
Abstract
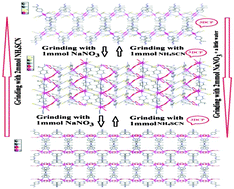
Reversible anion-exchange of nonporous 3D lead(II) coordination polymers with ligand 2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene (4-bpdh), from [Pb(4-bpdh)(μ-NCS)2]n (1) to intermediate 3D [Pb(4-bpdh)(μ-NCS)(NO3)]n (2) and then porous 3D coordination polymers of [Pb(4-bpdh)(NO3)2(H2O)]n (3) and [Pb(4-bpdh)(NO3)2]n (4) by solid state anion-replacement processes under mechanochemical reactions, have been studied. The reversible solid state structural transformations of compound 1 to compound 2 and then 3 and 4 by anion-replacement have been verified by PXRD and IR spectroscopy. Pb5O4SO4, Pb2(SO4)O and PbO nanoparticles were obtained by thermal decomposition of compounds 1, 2 and 3, 4 at 600 °C under an air atmosphere, respectively. These nanoparticles were characterized by powder X-ray diffraction (PXRD) and scanning electron microscopy (SEM).

 Please wait while we load your content...
Please wait while we load your content...