A highly selective colorimetric chemosensor for detection of nickel ions in aqueous solution†
Abstract
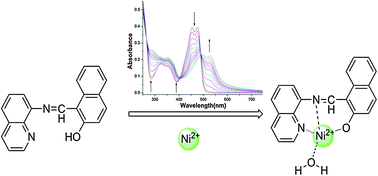
A highly selective chemosensor LX based on quinoline was described, which could instantly detect Ni2+ in aqueous solution with specific selectivity and high sensitivity. The addition of Ni2+ to sensor LX induced a remarkable color change from yellow to red; this sensing procedure could not be interfered by other coexistent competitive cations such as Fe3+, Co2+, and Cu2+. Thus LX could be used as a potential Ni2+ colorimetric and naked-eye chemosensor. Moreover, test strips based on sensor LX were fabricated, which could act as a convenient and efficient Ni2+ test for “in-the-field” measurement of Ni2+.

 Please wait while we load your content...
Please wait while we load your content...