Selective fluorescence sensing of salicylic acid using a simple pyrene appended imidazole receptor†
Abstract
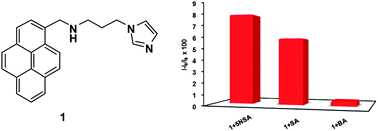
A simple salicylic acid selective fluorescence receptor 1 was designed by combining 1-pyrenecarboxaldehyde and 1-(3-aminopropyl)imidazole. The selective sensing of salicylic acid resulted in a significant increase in monomer emissions due to the π–π interactions between the benzene and pyrene rings. The nature of the interactions between receptor 1 and salicylic acid was investigated further by 1H NMR spectroscopy, and the energy minimised structure of the complex between receptor 1 and salicylic acid was optimised. Receptor 1 showed the highest binding constant with 5-nitrosalicylic acid (Ka = 7.18 × 104 M−1) among all the aromatic carboxylic acids tested. 5-Nitrosalicylic acid formed a complex with receptor 1 at a 1 : 1 ratio in EtOH.

 Please wait while we load your content...
Please wait while we load your content...