Ultra-sensitive and selective Hg2+ chemosensors derived from substituted 8-hydroxyquinoline analogues†
Abstract
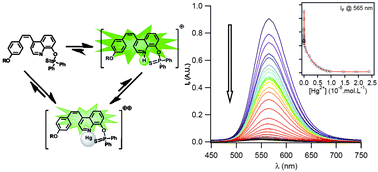
Novel analogues of 8-hydroxyquinoline with phosphinate or thiophosphinate functions and styryl fluorophores in the para position to the nitrogen atom were prepared via multi-step syntheses, using phosphorylation and Wittig coupling reactions. A strong affinity between the quinoline analogues and heavy metal ions such as Pb2+, Cd2+ and Hg2+ was highlighted. The interaction of the metal ions with the nitrogen of the styrylquinoline leads to a large red shift of the absorption and emission spectra in agreement with an increase of the photoinduced charge transfer character of the styryl fluorophore. In the presence of metal ions the appearance of a green fluorescence emission is also observed upon excitation at 420 nm or 840 nm, thanks to a significant increase of the two-photon response. Under optimal conditions, a mercury concentration of 15 ppt in a partially aqueous medium can be detected using the thiophosphinate derivative without interference from other metal ions.

 Please wait while we load your content...
Please wait while we load your content...