C–C bond formation via 1,2-addition of a tert-butylzinc reagent and carbonyls across conjugated dienes†
Abstract
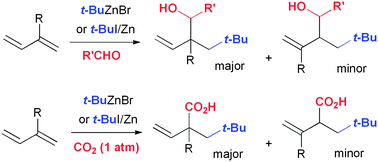
A mixture of t-butylzinc halide and an aldehyde reacts with conjugated dienes to provide 2-neopentyl homoallyl alcohols in high yields by 1,2-addition. Without the aldehyde, under carbon dioxide atmospheric pressure, the three components of t-butylzinc halide, butadiene, and carbon dioxide combine in a 1 : 1 : 1 ratio to give 2-neopentyl-3-butenoic acid in excellent yield.

 Please wait while we load your content...
Please wait while we load your content...