Preparation, characterization, and highly effective mercury adsorption of l-cysteine-functionalized mesoporous silica
Abstract
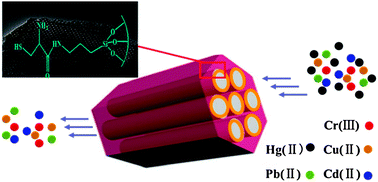
A novel L-cysteine-functionalized mesoporous SBA-15 (SH-SBA-15) material was synthesized by an easy two-step post-grafting method of L-cysteine using poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) as a template under acidic conditions. The material maintained its well-ordered mesostructure after two-step modification as confirmed by powder X-ray diffraction and transmission electron microscopy analyses. X-ray photoelectron spectroscopy and 13C cross-polarization/magic angle spinning NMR revealed that L-cysteine was successfully introduced into SBA-15. The material showed excellent mercury adsorption capacity (429 mg g−1) at room temperature and mercury uptake time (<5 min). Adsorption isotherms and uptake kinetics were also investigated. Equilibrium data were found to be represented better by the Freundlich isotherm model than the Langmuir one. The pseudo-second-order kinetics model best described the kinetic adsorption process of Hg(II) ions onto SH-SBA-15. Moreover, the material possessed high selectivity for mercury and can be easily eluted with 5% thiourea in 1 mol L−1 aqueous HCl.

 Please wait while we load your content...
Please wait while we load your content...