Synthesis and characterization of CaTiO3 particles with controlled shape and size
Abstract
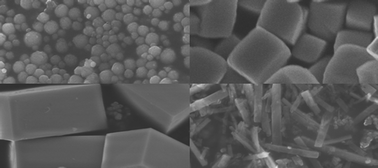
CaTiO3 particles with different morphologies and sizes are synthesized by mixing calcium nitrate and tetrabutyl titanate (Ti(OC4H9)4, TNB) into PEG [poly(ethylene glycol)] with varying water content. Electron microscopy observations reveal that the experimental conditions, such as the temperature of the suspension, NaOH concentration, reaction time and water content, can influence the shape and size of the CaTiO3 particles. The photocatalytic results show that the CaTiO3 rectangular particles have the highest photocatalytic activity.

 Please wait while we load your content...
Please wait while we load your content...