Synthesis, characterization and enhanced gas sensing performance of WO3 nanotube bundles†
Abstract
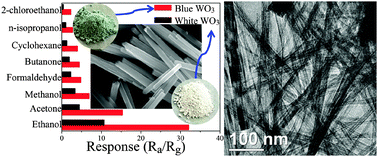
Well-defined WO3 nanotube bundles have been achieved via a facile hydrothermal method in a mixed solvent of ethylene glycol (EG) and water. The as-obtained products were systematically characterized by X-ray powder diffraction (XRD), X-ray photoelectron spectra (XPS), photoluminescence (PL), UV-vis diffuse reflectance spectroscopy, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and energy dispersive X-ray spectroscopy (EDS), respectively. The results indicate that the products consist of hierarchically nanostructured bundles assembled with smaller nanotube subunits. Meanwhile, the photoluminescence spectra demonstrate that the obtained products contain more oxygen vacancies compared with the reported WO3 materials. The morphologies of the products are found to be strongly dependent on the reaction time and the EG/H2O volume ratio, uniform WO3 nanotube bundles can be fabricated readily just via tuning the volume ratio of EG and H2O. Through a series of time-dependent morphological evolution studies, the growth process of WO3 nanostructures has been investigated, and the corresponding growth mechanism is discussed in detail as well. Additionally, the gas-sensing performances of uniform WO3 nanotube bundles were investigated for 100 ppm of ethanol. It was found that the WO3 nanotube bundles sensor displayed significantly better sensing performance than many reported WO3 materials due to more electron donor related oxygen vacancies.

 Please wait while we load your content...
Please wait while we load your content...