Metal–organic frameworks constructed from flexible ditopic ligands: conformational diversity of an aliphatic ligand†
Abstract
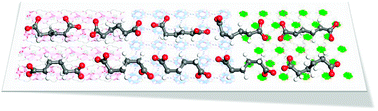
The solvothermal reaction of adipic acid as a flexible ditopic ligand and the metal ions MnII, CoII, and TbIII afforded three novel metal–organic frameworks (MOFs), {[Mn2(adipate)2(DMA)]} (1), {[Co2(adipate)2(DMF)]·1DMF·1.5H2O} (2), and {[Tb3(adipate)4.5(DMF)2]} (3) (DMA = N,N-dimethylacetamide; DMF = N,N-dimethylformamide), respectively, which were structurally characterized by single-crystal X-ray diffraction. Depending on the kind of metal ion and solvent system, the conformations and coordination modes of the adipate ligands were diverse and governed the entire MOF structure. Compound 1 consists of the secondary building units (SBUs) of Mn–O chains that were linked by adipate ligands extending in two-dimensional sheets, which were infinitely stacked in a layer-by-layer manner. Compound 2 presented a three-dimensional MOF constructed from Co–O chains and bridging adipate ligands extending in four different directions. Compound 3 also had a three-dimensional structure which was formed by Tb–O chains connected with adipate ligands in six directions. From these structures, ten different adipate ligands with diverse conformations were found, and the potential energy of each conformation was calculated by the first-principles density function. In addition, the luminescence properties of the Tb-based MOF 3 were investigated in the solid state at room temperature.

 Please wait while we load your content...
Please wait while we load your content...