Water-controlled chiral inversion of a nitrogen atom during the synthesis of diaziridines from α-branched N,N'-dialkyl α-diimines†
Abstract
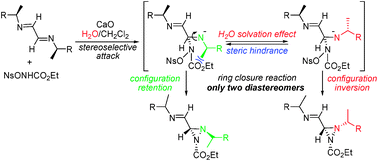
Desymmetrization of chiral α-diimines by ethyl nosyloxycarbamate using water as a co-solvent takes place with very high diastereoselectivity, the nucleophilic attack occurring only on the less hindered side of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. Interestingly, the presence of water in the reaction medium likely stabilizes the anion intermediate slowing down the successive cyclization reaction and favoring the rotation around the C–N single bond. In fact, as confirmed by ROESY experiments, only the two diastereomeric 3-(iminomethyl)diaziridine-1-carboxylates that differ in the absolute configuration of the alkyl substituted nitrogen atoms were always obtained in equimolar ratios. Finally, all compounds were easily obtained in optically pure form through HPLC separation and can be considered as interesting chiral synthons.
N double bond. Interestingly, the presence of water in the reaction medium likely stabilizes the anion intermediate slowing down the successive cyclization reaction and favoring the rotation around the C–N single bond. In fact, as confirmed by ROESY experiments, only the two diastereomeric 3-(iminomethyl)diaziridine-1-carboxylates that differ in the absolute configuration of the alkyl substituted nitrogen atoms were always obtained in equimolar ratios. Finally, all compounds were easily obtained in optically pure form through HPLC separation and can be considered as interesting chiral synthons.

 Please wait while we load your content...
Please wait while we load your content...