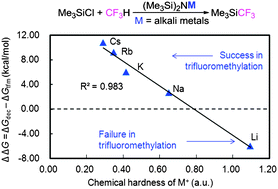
Prakash and co-workers recently reported a direct trifluoromethylation of Si, B, S, and C centers using fluoroform (CF3H) and dramatic effects of alkali metal salts of hexamethyldisilazane in this reaction (Science, 2012, 338, 1324). Herein, the detailed mechanisms of trifluoromethylation of Si and C centers in the presence of (Me3Si)2NK as a base have been studied using the DFT method. It has been found that the origin of the dramatic effect of the alkali metals is the stability of an intermediate MCF3. More interestingly, a linear relationship has been found between the chemical hardness of M+ (M = Li, Na, K, Rb, Cs) and the difference between the values of ΔGdec and ΔGtfm (ΔGdec, decomposition energy of the key intermediate MCF3; ΔGtfm, relative energy barrier for the formation of a Si–CF3 or C–CF3 bond). These results may help to both theoretically and experimentally search for better bases to develop more atom-economic and environmentally benign protocols to achieve trifluoromethylation using CF3H.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...