A new way to tune relative humidity: by saturated ionic liquid aqueous solutions
Abstract
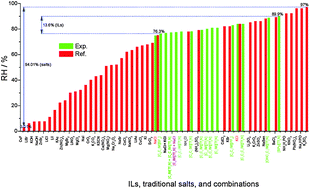
In this paper, a new way to control the relative humidity (RH) by hydrophobic ionic liquids (ILs) was proposed. The influences of cation type, anion type, alkyl chain length, substitution of the cation ring, functionalization, and combinations of ILs on the RH were investigated. The results showed that cation ring, functionalization and temperature had a significant influence on the RH of saturated IL aqueous solutions, while the influence of alkyl chain length was negligible. Then, a repulsion-induced mechanism was proposed to qualitatively explain the RH of saturated IL aqueous solutions. Simply speaking, ILs with high hydrophilicity induce water molecules from the air, thus resulting in a low RH, and vice versa.

 Please wait while we load your content...
Please wait while we load your content...