In situ enzymatic generation of H2O2 from O2 for use in oxidative bleaching and catalysis by TAML activators†
Abstract
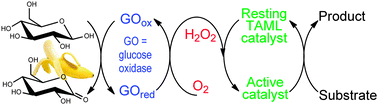
Iron-TAML activators of peroxides are functional mimics of peroxidase and short-circuited cytochrome P450 enzymes that perform numerous transformations that appear by all measures to date to be fully life cycle compatible with the environment. Here we show how to design the same catalytic chemistry without the direct addition of H2O2, thereby removing the need to transport and store hydrogen peroxide. In this new approach, dioxygen is reduced in situ by D-glucose in the presence of glucose oxidase (GO) from Aspergillus niger. The resulting tandem TAML–GO system exhibits similar catalytic efficiency to the TAML/H2O2 prototype. The tandem system is shown here to efficiently decolorize Orange II and oxidize NADH, both near neutral pH (7.5). Computational simulations of the kinetic data suggest that denaturing of GO by oxidized TAML intermediates does not occur throughout each process. This tandem system brings the advantage of in situ generation of low concentrations of H2O2 during the catalytic cycles. This minimizes both unproductive H2O2 consumption resulting from the catalase-like activity of iron TAMLs and suicidal inactivation. Kinetic data for oxidation of NADH by TAML/H2O2 are also reported.
- This article is part of the themed collection: In honour of Bernard Meunier

 Please wait while we load your content...
Please wait while we load your content...