Density functional theory (DFT)/time dependent density functional theory (TDDFT) calculations combined with the effective fragment potential (EFP) method have been carried out to study the electronic structure and the excited state properties of 6-aminocoumarin (6AC) with five water molecules (6AC–(H2O)5 complex). Ground-state geometries are optimized using DFT with the B3LYP functional combined with cc-pVDZ basis sets and transition energies are computed with the same basis set and functional. One intermolecular hydrogen bond (HB) N⋯H–O (type A) is formed between the amino group of 6AC and one water molecule, two intermolecular HBs C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O (type B) are formed between the carbonyl group of 6AC and two water molecules, and two HBs of N–H⋯O (type C), via participation of their amino hydrogen atoms, with the oxygen atoms of water molecules. The change in hydrogen bond energy, ΔEHB of the 6AC–(H2O)5 molecule and ΔEHB for each HB of the 6AC–(H2O)5 molecule are calculated separately. Upon excitation of the 6AC–(H2O)5 complex, the A type HB is weakened with a decrease of 11.03 kJ mol−1 energy, whereas B and C type HBs are strengthened with increases of 5.28 and 18.14 kJ mol−1 energy, respectively. In this theoretical work, ΔEHB is calculated using the procedure proposed by T. Nagata et al. to calculate solute–solvent interaction energy in J. Chem. Phys., 2011, 134, 034110 and this study also confirmed again that intermolecular hydrogen bonds between the 6AC chromophore and aqueous solvents are strengthened, not cleaved upon electronic excitation, which is in accordance with Zhao's works (2011 Wiley Periodicals, Inc. J. Comput. Chem., 2011, 32, 545).
O⋯H–O (type B) are formed between the carbonyl group of 6AC and two water molecules, and two HBs of N–H⋯O (type C), via participation of their amino hydrogen atoms, with the oxygen atoms of water molecules. The change in hydrogen bond energy, ΔEHB of the 6AC–(H2O)5 molecule and ΔEHB for each HB of the 6AC–(H2O)5 molecule are calculated separately. Upon excitation of the 6AC–(H2O)5 complex, the A type HB is weakened with a decrease of 11.03 kJ mol−1 energy, whereas B and C type HBs are strengthened with increases of 5.28 and 18.14 kJ mol−1 energy, respectively. In this theoretical work, ΔEHB is calculated using the procedure proposed by T. Nagata et al. to calculate solute–solvent interaction energy in J. Chem. Phys., 2011, 134, 034110 and this study also confirmed again that intermolecular hydrogen bonds between the 6AC chromophore and aqueous solvents are strengthened, not cleaved upon electronic excitation, which is in accordance with Zhao's works (2011 Wiley Periodicals, Inc. J. Comput. Chem., 2011, 32, 545).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O (type B) are formed between the
O⋯H–O (type B) are formed between the 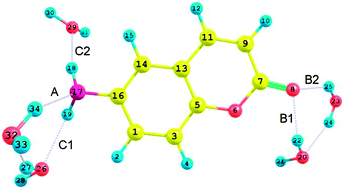

 Please wait while we load your content...
Please wait while we load your content...