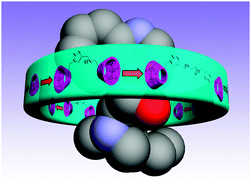
Three bridged bis(β-cyclodextrin)s 3–5 possessing 1,2,3-triazole moieties and polyether chains of different lengths have been synthesized by click reactions in high yields. Their binding affinities towards four cinchona alkaloids, namely, cinchonine, cinchonidine, quinine, and quinidine, have been quantitatively investigated by means of spectrophotometric titrations and 2D NMR spectroscopy. Spectroscopic analyses demonstrated that, compared with native β- and γ-cyclodextrins, bridged bis(β-cyclodextrin)s with a rigid spacer give enhanced molecular binding abilities towards the selected substrates. Moreover, the factors resulting in the significant differences in photophysical behaviors of bridged bis(β-cyclodextrin)s towards cinchona alkaloids are discussed from the viewpoint of the binding geometry of host–guest complexes, revealing that the aromatic ring containing the nitrogen atom of quinine is accommodated in the cavity of 3, whereas the rings of cinchonine, cinchonidine, and quinidine are located out of the cavity of the cyclodextrin and are exposed to aqueous media.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...