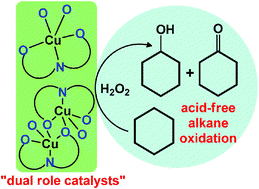
The aquasoluble [Cu(H2O)((CH3)2NCHO)(HL)] (2) and [Cu2(CH3OH)2(μ-HL)2] (3) CuII complexes were prepared by reaction of CuII nitrate hydrate with the new 3-(2-hydroxy-4-carboxyphenylhydrazone)pentane-2,4-dione (H3L, 1), in the presence (for 2) or absence (for 3) of (n-C4H9)2SnO, and characterized by elemental analysis, IR spectroscopy and X-ray single crystal diffraction. Magnetic susceptibility measurements, in compound 3, reveal strong antiferromagnetic coupling between the CuII ions through the μ2-phenoxido-O atoms, J = −203(1) cm−1. Complexes 2 and 3 act as catalyst precursors for the acid-free peroxidative oxidation of cyclohexane to the mixture of cyclohexyl hydroperoxide (primary product), cyclohexanol and cyclohexanone (TONs and yields up to 163 and 14.4%, respectively), as well as for the selective aerobic oxidation of benzyl alcohols to benzaldehydes in aqueous solution, mediated by a TEMPO radical, under mild conditions (TONs and yields up to 390 and 94%, respectively). In the alkane oxidations, 2 and 3 appear to behave as “dual role catalysts” combining, in one molecule, an active metal centre and an acidic promoting group, to provide a high activity of the system even without any acid promoter.

 Please wait while we load your content...
Please wait while we load your content...