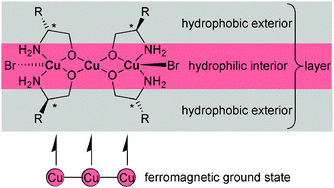
The trinuclear secondary building unit (SBU) {Cu3(μ-L)4X2} constructed from chelating and alcoholate-O-bridging chiral amino alcoholate (amino alkoxido, μ-L) and terminal halide ligands (X) gives rise to two-dimensional (2D) homochiral supramolecular polymers through the bridging action of the halide ligand. Compounds 2D-[Cu3(μ-L)4(μ3-X)2] {X = Br (1 to 4), X = Cl (5, 6); L = amino-ethanolate (1, 5), (R)-2-amino-propan-1-olate (2), (R)-2-amino-butan-1-olate (ab) (3, 6), (R)-2-amino-2-phenyl-ethanolate (4)} were synthesized from 2-amino alcohol, LH with CuBr2 or CuCl2, respectively, in the presence of triethylamine (TEA). The crystal packing of isomorphous 2D-[Cu3(μ-L)4(μ3-X)2] shows a separation of the hydrophobic alkyl from the hydrophilic amino-/alcoholate-/Cu–Br-region. Charge-assisted Cu(2+)⋯(−)Br- and hydrogen-bonding interactions in the hydrophilic region are the driving force of “hydrophobic exterior layer” formation with a hydrophilic interior exposing the hydrophobic alkyl groups to the exterior. Stacking of the layers through weak van-der-Waals interactions between the alkyl groups correlates with formation of thin crystal plates along the stacking direction. A lower ligand concentration yields both dinuclear and mononuclear SBUs in one-dimensional (1D-) homochiral coordination polymers 1D-[{Cu2(μ-ab)2Br2}2{(μ3-Br)Cu(abH)2Br}{(μ3-Br)Cu(abH)(CH3OH)Br}] (7) and 1D-[{Cu2(μ-ab)2Cl2}2{(μ3-Cl)Cu(abH)(CH3CH2OH)Cl}2] (8). In {[Cu(rac-abH)(rac-ab)H2O]ClO4}2 (9) two SBUs combine through charge assisted H-bonds to a dimeric unit. Temperature dependent magnetic susceptibility measurements of 1 and 3 show ferromagnetic coupling but no magnetic ordering. However, at temperatures lower than 3 K an onset of long-range magnetic ordering can be observed. AC magnetic measurements in the range of 1.9 K to 5 K at different frequencies show that the long range magnetic ordering is achieved at lower temperatures than that. Correlation of magnetic coupling with the Cu–O–Cu angle is in agreement with the structural parameters of 1 and 3. Very good agreement between experimental and DFT calculated J,j coupling constants shows that the interaction between the terminal copper(II) ions in these linear trinuclear SBUs is operative and it cannot be neglected.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...