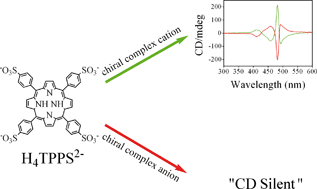
The non-covalent interactions of chiral metal complexes with the achiral 5,10,15,20-tetrakis (4-sulonatophenyl) porphyrin (H4TTPS2−) have been investigated by UV-vis and circular dichroism (CD) spectra. The results show that under acidic environments, only the chiral complex cations ([CoBr(NH3)(en)2]+, [Co(en)3]3+, [Ru(phen)3]2+) could interact with H4TTPS2− to form chiral aggregates, accompanied with the metal-centered chirality information transferred to the formed J-aggregates. However, the chiral complex anion ([Co(edta)]−) does not cause the self-assembly process. The competitive binding interactions between an achiral water-soluble cationic surfactant (N-hexadecyltrimethyl ammonium chloride, CTAC) and a cationic polyelectrolyte (polyallylamine, PAA) with the chiral metal complex H4TTPS2− J-aggregates, respectively, were also investigated. It was found that chiral-symmetry-breaking phenomena occur in the cationic surfactant induced event. In the case of a cationic polyelectrolyte, it could change the conformational flexibility of the H4TTPS2− aggregates. These results may lead us to understand the possible mechanism of the supramolecular self-assembly process by the non-covalent interactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...