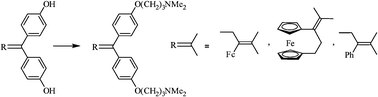
We have prepared several organometallic systems whose structures are closely analogous to that of tamoxifen, the drug used in the treatment of hormone-dependent breast cancers, but which now possess two basic aminoalkyl chains: O(CH2)3NMe2. Despite the absence of a phenolic functionality, these ferrocenyl compounds 3, 4 and their organic analogue 5 recognize the estrogen receptor but in addition exhibit strong antiproliferative effects on hormone-dependent breast cancer cells (MCF-7), and also on hormone-independent ones (MDA-MB-231) with, in this case, an IC50 value of about 0.4 μM. The ferrocenyl moiety does not create a major effect here compared to a purely organic aromatic group. On the other hand, the presence within the molecule of two vicinal basic entities, potentially allowing complexation to metal ions such as Zn2+, could perhaps be the key to the antiproliferative effectiveness of this series which operates via a different mechanism to that of hydroxytamoxifen 1 and hydroxyferrocifen 2. The behaviour of these new species is discussed. They possess the distinctive feature of combining a strong antiproliferative effect with intense antibacterial and antifungal activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...