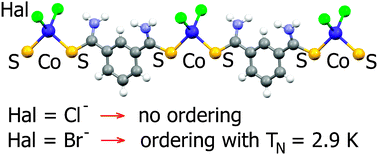
A series of 1D coordination polymers [Co(m-dtab)Cl2]n (1), [Co(m-dtab)Br2]n (2) and [Ni(m-dtab)2(Br)2]n (3), where m-dtab = the dithioamide of 1,3-benzenedicarboxylic acid, were prepared. The structures of all complexes were determined by X-ray diffraction. Magnetic properties of the compounds were characterized by molecular susceptibility vs. T dependence in the temperature range from 2 to 300 K. All compounds possess antiferromagnetic exchange interactions, and antiferromagnetic ordering was found in [Co(m-dtab)Br2]n and [Ni(m-dtab)2Br2]n at TN = 2.9 K and 2.6 K, respectively. DFT calculations showed that exchange interactions in [Co(m-dtab)(Hal)2]n could be transferred through two pathways: m-dtab between metal ions or interchain π–π stacking of aromatic rings, so the systems are not 1D from the viewpoint of magnetochemistry. The results of DFT calculations are consistent with the existence of magnetic ordering.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...