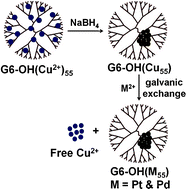
In this report we present the synthesis and characterization of Pt and Pd dendrimer-encapsulated nanoparticles (DENs) using the method of galvanic exchange. Sixth-generation hydroxyl-terminated poly(amidoamine) dendrimers were used to prepare Cu DENs composed of 55 atoms. In the presence of either PtCl42− or PdCl42−, the less noble Cu DENs oxidize to Cu2+ leaving behind an equal-sized DEN of Pt or Pd, respectively. DENs prepared by direct reduction with BH4−, which is the common synthetic route, and those prepared by galvanic exchange have the same composition, structure, and properties as judged by UV-vis spectroscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, X-ray absorption spectroscopy, and electrochemical methods. However, the galvanic exchange synthesis is much faster (3 h vs. 96 h), and the yield of reduced DENs is significantly higher (nearly 100% in the case of galvanic exchange).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...