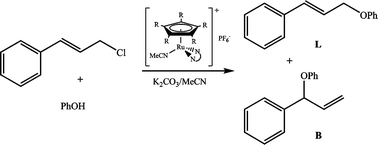
The different behaviours of Ru(η5-C5H5) and Ru(η5-C5Me5) precatalysts, [Ru(η5-C5R5)(NCMe)(N,N)]PF6 (R = H, Me), in the allylic etherification reaction of cinnamyl chloride using the phenoxide anion as a nucleophile was considered. The N,N ligands are the commercial products 1,8-diaminonaphthalene and 8-aminoquinoline, and their derivatives obtained by alkylation of the amino nitrogen atoms: alkyl substituents that are also bulky chiral C2-symmetric frameworks allow modulation of the basicity and steric demand of the ligands. Some of the precatalysts, [Ru(η5-C5R5)(NCMe)(N,N)]PF6 (R = H, Me), were also synthesized and characterized. The cinnamyl phenyl ether isomers were obtained with very high B/L regioselectivity values, either with Ru(η5-C5H5) or Ru(η5-C5Me5) precatalysts. The highest B/L regioselectivity values achieved with Ru(η5-C5Me5) precatalysts were found with the N,N ligand 1,8-diaminonaphthalene and its derivatives; with Ru(η5-C5H5) precatalysts best B/L values were obtained with ligands derived from 8-aminoquinoline. A correlation between the B/L regioselectivity, and the σ-donor power and bulkiness of the substituents at the nitrogen atoms of the N,N coordinated ligand was established, but the Ru(η5-C5H5) or Ru(η5-C5Me5) precatalysts followed an opposite trend. It was also found that the low ee values did not depend on the diastereomeric composition of the chiral-at-metal precatalyst [Ru(η5-C5R5)(NCMe)(N,N)]PF6.

 Please wait while we load your content...
Please wait while we load your content...