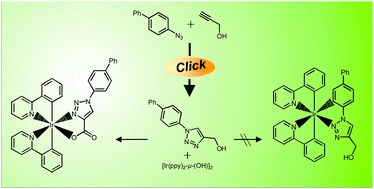
Two different procedures of the ‘click’ reaction were applied to synthesize a library of 1-aryl- and 4-aryl-functionalized 1H-[1,2,3]triazoles as new ligands for phosphorescent iridium(III) complexes. For three examples, single crystal X-ray analysis was carried out and the structural properties were discussed. The reactive μ-dihydroxy-bridged iridium(III) precursor complex [(ppy)2Ir-μ-(OH)]2 (ppy = 2-phenylpyridinato) was prepared for the complexation of the herein described ligands. During these complexation studies, an unexpected metal-assisted oxidation pathway was observed for the hydroxymethyl-substituted 1-aryl-1H-[1,2,3]triazoles 2d–f leading selectively to a [carboxylate-N3,O]-coordination of the ligands to the iridium(III) centers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...