Crystal structures and sodium/silver distributions within the ionic conductors Na5Ag2Fe3(As2O7)4 and Na2Ag5Fe3(P2O7)4†
Abstract
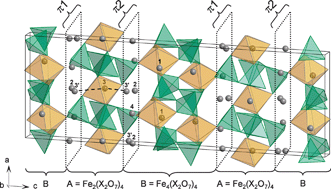
The crystal structures of new Na5Ag2Fe3(As2O7)4 and Na2Ag5Fe3(P2O7)4 compounds, prepared through ion exchange from Na7Fe3(X2O7)4 (X =

 Please wait while we load your content...
Please wait while we load your content...