The photochromic and self-assembling properties of diarylethenes having chiral amphiphilic chains at the reactive carbon atoms†
Abstract
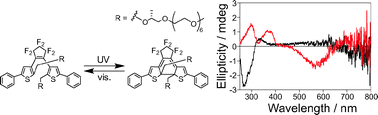
A photochromic diarylethene derivative having chiral polyethylene glycol side chains at the reactive carbon atoms was synthesized. The diarylethene showed a photochromic reaction upon irradiation with UV and visible light in both
- This article is part of the themed collection: New Horizons of Photochromism

 Please wait while we load your content...
Please wait while we load your content...