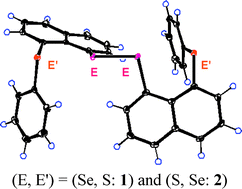
The structure, stability and reactivity of E′⋯E–E⋯E′ (E2E′2) 4c–6e are examined employing naphthalene 1,8-positions in 1-(8-PhE′C10H6)EE(C10H6E′Ph-8′)-1′ [1 (E = Se, E′ = S), 2 (E = S, E′ = Se), 3 (E = E′ = Se) and 4 (E = E′ = S)], together with 1-C10H7EEC10H7-1′ [5 (E = Se) and 6 (E = S)]. Linear alignments of four Se2S2 atoms in 1 and 2 are confirmed by the X-ray analysis. 1 was not reduced by sodium borohydride, whereas 2 was, contrary to the expectation. Similarly, E–E in 3, 5 and 6 were cleaved, whereas that in 4 was not, when allowed to react with NaBH4 in aqueous THF. The reactivity of the E–E bonds in E2E′2 in 1–4 is not controlled by the central E atoms but by the outside E′ atoms. Quantum chemical (QC) calculations are performed on 1-(8-MeE′C10H6)EE(C10H6E′Me-8′)-1′ (13–16: models of 1–4, respectively), 5 and 6, together with the related species. Conformers having E2E′2 4c–6e (abbreviated by BA) are the global minima for 13, 15 and 16, which reproduces the observed structures: BA must be stabilized by the formation of E2E′2 4c–6e. 14 (AB) with double n(S)⋯σ*(Se–C) 3c–4e interactions is the global minimum, which shows that σ*(Se–C) plays a crucial role to stabilize the 3c–4e in AB. The novel reactivity of E–E is considered based on the QC calculations.

 Please wait while we load your content...
Please wait while we load your content...