Effects of the alkyl substituent in the π-donor heteroatom on the kinetic and thermodynamic acidities of Fischer thiocarbene complexes†
Abstract
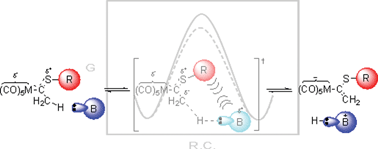
Rate constants for the proton transfer reaction from Fischer thiocarbene complexes (CO)5M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(SR1)CH3 (M = Cr or W; R1 = n-butyl,
C(SR1)CH3 (M = Cr or W; R1 = n-butyl,

 Please wait while we load your content...
Please wait while we load your content...