Synthesis and characterisation of O-6-alkylthio- and perfluoroalkylpropanethio-α-cyclodextrins and their O-2-, O-3-methylated analogues
Abstract
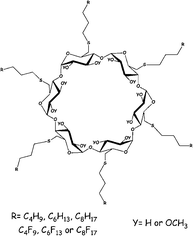
The synthesis of twelve alkylthio- or perfluoroalkylpropanethio-α-cyclodextrin derivatives and their O-2-, O-3-methylated analogues are described. The

 Please wait while we load your content...
Please wait while we load your content...