Synthesis and complexation study of (1,4-linked)-homothiaisocalixnaphthalenes†
Abstract
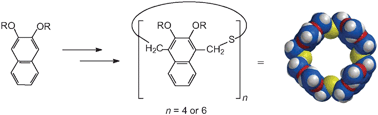
A series of new cavity-containing molecular receptors, “homothiaisocalixnaphthalenes” containing 2,3-dialkoxy-substituted naphthalene units, have been synthesized, and some of their complexation properties have been investigated. The syntheses of the octaethoxy- and n-octapropoxy-octahomotetrathiaisocalix[4]naphthalenes were accompanied by small amounts of the corresponding higher dodecahomohexathia homologues. All of the

 Please wait while we load your content...
Please wait while we load your content...