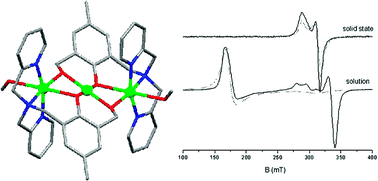
A linear trinuclear copper(II) complex containing phenoxido- and alkoxido-bridges between the metal centers has been isolated and structurally characterized. The complex cation consists of a linear array of three copper ions, assembled by means of two doubly deprotonated ligands. The octahedral coordination sphere of the two peripheral copper(II) ions is completed by weakly bound methanol molecules, and the square-planar central metal ion is located on an exact, crystallographic inversion center. Temperature-dependent magnetic susceptibility studies reveal the presence of antiferromagnetic exchange coupling between the copper(II) ions in the trinuclear unit along with small intermolecular antiferromagnetic interactions in the low temperature range. The results were fitted in two different ways, (i) taking into account solely the exchange interaction between the adjacent metal centers or, (ii) regarding exchange interactions between both adjacent and non-adjacent copper(II) ions. Solid-state temperature-dependent X-band EPR studies in the range 4.2–250 K indicate a doublet ground spin state |½, 1〉. In solution, the ground spin state of the complex is found to be a quartet (S = ![[fraction three-over-two]](https://www.rsc.org/images/entities/char_e0ee.gif) ), suggesting a modification of the exchange coupling interactions between the copper(II) ions. The simulation of the 4.2 K solution spectrum gives rise to the best parameters D > 0.8 cm−1, g⊥ = 2.04 and g∥ = 2.21.
), suggesting a modification of the exchange coupling interactions between the copper(II) ions. The simulation of the 4.2 K solution spectrum gives rise to the best parameters D > 0.8 cm−1, g⊥ = 2.04 and g∥ = 2.21.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[fraction three-over-two]](https://www.rsc.org/images/entities/char_e0ee.gif) ), suggesting a modification of the exchange coupling interactions between the copper(II) ions. The simulation of the 4.2 K solution
), suggesting a modification of the exchange coupling interactions between the copper(II) ions. The simulation of the 4.2 K solution 

 Please wait while we load your content...
Please wait while we load your content...