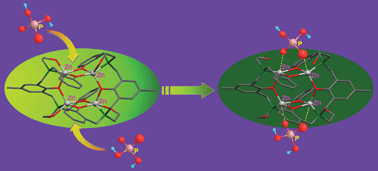
A cresolic oxygen bridging ligand, 2,6-bis{[(2-hydroxybenzyl)(2-hydroxyethyl)amino]methyl}-4-methylphenol (L), has been synthesized and characterized. The coordination of ZnII ions with L gives a novel tetranuclear complex, [ZnII4(L−3H)2](ClO4)2·3.5H2O (I), which crystallizes in a triclinic system with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.261(5) Å, b = 13.887(6) Å, c = 20.738(8) Å, α = 89.996(7)°, β = 88.163(7)° and γ = 85.016(7)°. The cationic core of I is formed by four ZnII cations bridged by two cresolic and four phenolic oxygen atoms from two ligands. All four ZnII centers are pentacoordinated and adopt a distorted square pyramid geometry. ESMS and 1H NMR data indicate that I is stable in solution, and the fluorescence measurement demonstrates that it has strong fluorescence at λex = 298 nm. The fluorescent complex can selectively sense the dihydrogen phosphate anion in methanol, and the binding phenomenon can be monitored via UV-vis absorption changes and fluorescence quenching effects. The potential binding mode of I with H2PO4− has been studied by ESMS and 1H NMR spectroscopy.
, a = 12.261(5) Å, b = 13.887(6) Å, c = 20.738(8) Å, α = 89.996(7)°, β = 88.163(7)° and γ = 85.016(7)°. The cationic core of I is formed by four ZnII cations bridged by two cresolic and four phenolic oxygen atoms from two ligands. All four ZnII centers are pentacoordinated and adopt a distorted square pyramid geometry. ESMS and 1H NMR data indicate that I is stable in solution, and the fluorescence measurement demonstrates that it has strong fluorescence at λex = 298 nm. The fluorescent complex can selectively sense the dihydrogen phosphate anion in methanol, and the binding phenomenon can be monitored via UV-vis absorption changes and fluorescence quenching effects. The potential binding mode of I with H2PO4− has been studied by ESMS and 1H NMR spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.261(5) Å, b = 13.887(6) Å, c = 20.738(8) Å, α = 89.996(7)°, β = 88.163(7)° and γ = 85.016(7)°. The cationic core of I is formed by four ZnII cations bridged by two cresolic and four phenolic oxygen atoms from two
, a = 12.261(5) Å, b = 13.887(6) Å, c = 20.738(8) Å, α = 89.996(7)°, β = 88.163(7)° and γ = 85.016(7)°. The cationic core of I is formed by four ZnII cations bridged by two cresolic and four phenolic oxygen atoms from two 

 Please wait while we load your content...
Please wait while we load your content...